Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yamaguchi, Tetsuji
Kyouto Daigaku Daigakuin Kougaku Kenkyuka Hakase Gakui Ronbun, 136 Pages, 2001/01
no abstracts in English
; Ueno, Fumiyoshi
JNC TN9400 2000-017, 10 Pages, 2000/03
lt is difficult to get hold the behave of growth of cavity which nucleates in grain boudaly in experimental observation. lt is considerd that numerical simulation is effective for the grasp of behave of cavity growth, because it is able to grasp the microscopic behavior of internal material whici is hardly observation. We examine the factor that the diffusive ratio and the stress etc., affected growth of cavity on grain boundary with numerical simulation using diffusive equation. As the result, a following knowledge was obtained. (1)With dominant of grain boundary diffusion, the shape of cavity transitions from quasi-equilibrium to crac-like. ln other hand, with dominant of surface diffusion, cavity grows up with initial shape. (2)With dominate of grain boundary diffusion, it accelerates the growth rate of the cavity near the tip by grain boundaly diffusion induced stressing perpendicular to gain boundary (3)With dominant of surface diffusion, the distribution of chemical potential is uniformity on cavity surface. ln other hand, with dominant of grain boundary diffusion compare to that of surface diffusion, the gradient of chemical potential is increased at the tip of cavity.
Sato, Haruo
JNC TN8400 99-062, 16 Pages, 1999/10
Effective diffusion coefficients (De) for Ni , Sm
, Sm , Am
, Am and SeO
and SeO were measured as a function of the ionic charge of diffusion species to quantitatively evaluate the effect of ionic charge in compacted bentonite. The De measurements for Ni
were measured as a function of the ionic charge of diffusion species to quantitatively evaluate the effect of ionic charge in compacted bentonite. The De measurements for Ni and Sm
and Sm were carried out for a bentonite dry density of 1.8 Mg
were carried out for a bentonite dry density of 1.8 Mg m
m with a simulated porewater condition of pH5
with a simulated porewater condition of pH5 6 by through-diffusion method. The De values for SeO
6 by through-diffusion method. The De values for SeO were measured for a bentonite dry density of 1.8 Mg
were measured for a bentonite dry density of 1.8 Mg m
m with a simulated porewater condition of pH11. The De measurements for Am
with a simulated porewater condition of pH11. The De measurements for Am were carried out for the dry densities of 0.8, 1.4 and l.8 Mg
were carried out for the dry densities of 0.8, 1.4 and l.8 Mg m
m with a porewater condition of pH2 in order to check cation exclusion. Sodium bentonite, Kunigel-V1 was used for those measurements. For the measurements of Am, H-typed Kunigel-V1 which interlayer ion (Na
with a porewater condition of pH2 in order to check cation exclusion. Sodium bentonite, Kunigel-V1 was used for those measurements. For the measurements of Am, H-typed Kunigel-V1 which interlayer ion (Na ) was exchanged with H
) was exchanged with H was used, because the experiments are carried out for a low pH range. The order of obtained De values was Sm
was used, because the experiments are carried out for a low pH range. The order of obtained De values was Sm
 Ni
Ni
 Am
Am
 SeO
SeO . These De values were compared to those reported to date. Consequently, the order of De values was Cs
. These De values were compared to those reported to date. Consequently, the order of De values was Cs
 Sm
Sm
 HTO
HTO  Ni
Ni
 anions (I
anions (I , Cl
, Cl , CO
, CO , SeO
, SeO TcO
TcO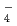 , NpO
, NpO CO
CO , UO
, UO (CO
(CO )
)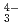 ), showing a tendency of cations
), showing a tendency of cations  HTO
HTO  anions. Only the De values of Am
anions. Only the De values of Am were approximately the same degree as those of anions. The reason that the De of Ni
were approximately the same degree as those of anions. The reason that the De of Ni was lower than that of HTO may be because the free water diffusion coefficient (Do) of Ni
was lower than that of HTO may be because the free water diffusion coefficient (Do) of Ni is about 1/3 of that of HTO. The cause that the De of Am
is about 1/3 of that of HTO. The cause that the De of Am was approximately the same degee as those of anions may be because the Do of Am
was approximately the same degee as those of anions may be because the Do of Am is about 1/3 of that of HTO and that Am
is about 1/3 of that of HTO and that Am was electrostatically repulsed from the surface of bentonite by cation exclusion. The formation factors (FF), calculated normalizing Do, were in the ...
was electrostatically repulsed from the surface of bentonite by cation exclusion. The formation factors (FF), calculated normalizing Do, were in the ...
 diffusion experiment in a granite from underground research laboratory in Manitoba, Canada
diffusion experiment in a granite from underground research laboratory in Manitoba, CanadaYamaguchi, Tetsuji; Nakayama, Shinichi; Vandergraaf, T. T.*
Materials Research Society Symposium Proceedings, Vol.506, p.1091 - 1093, 1998/00
no abstracts in English
Yamaguchi, Tetsuji; Sakamoto, Yoshiaki;
Journal of Nuclear Science and Technology, 30(8), p.796 - 803, 1993/08
Times Cited Count:17 Percentile:81.88(Nuclear Science & Technology)no abstracts in English